Oceans Becoming Acidic Ten Times Faster than Thought Before.
 The world’s oceans are turning acidic due to the buildup of carbon dioxide (CO2) in the atmosphere, and scientists say the effects on marine life will be catastrophic.
The world’s oceans are turning acidic due to the buildup of carbon dioxide (CO2) in the atmosphere, and scientists say the effects on marine life will be catastrophic.
“Increasing levels of carbon dioxide in the atmosphere may make Earth’s oceans more acidic faster than previously thought—unbalancing ecosystems in the process. New study says: Helen Scales for National Geographic News”
For tens of millions of years, Earth’s oceans have maintained a relatively stable acidity level. It’s within this steady environment that the rich and varied web of life in today’s seas has arisen and flourished. But research shows that this ancient balance is being undone by a recent and rapid drop in surface pH that could have devastating global consequences.
Since the beginning of the industrial revolution in the early 1800s, fossil fuel-powered machines have driven an unprecedented burst of human industry and advancement. The unfortunate consequence, however, has been the emission of billions of tons of carbon dioxide (CO2) and other greenhouse gases into Earth’s
atmosphere.
Scientists now know that about half of this anthropogenic, or man-made, CO2 has been absorbed over time by the oceans. This has benefited us by slowing the climate change these emissions would have instigated if they had remained in the air.
But relatively new research is finding that the introduction of massive amounts of CO2 into the seas is altering water chemistry and affecting the life cycles of many marine organisms, particularly those at the lower end of the food chain.
When carbon dioxide dissolves in this ocean, carbonic acid is formed. This leads to higher acidity, mainly near the surface, which has been proven to inhibit shell growth in marine animals and is suspected as a cause of reproductive disorders in some fish.
On the pH scale, which runs from 0 to 14, solutions with low numbers are considered acidic and those with higher numbers are basic. Seven is neutral. Over the past 300 million years, ocean pH has been slightly basic, averaging about 8.2. Today, it is around 8.1, a drop of 0.1 pH units, representing a 25-percent increase in acidity over the past two centuries. The oceans currently absorb about a third of human-created CO2 emissions, roughly 22 million tons a day.
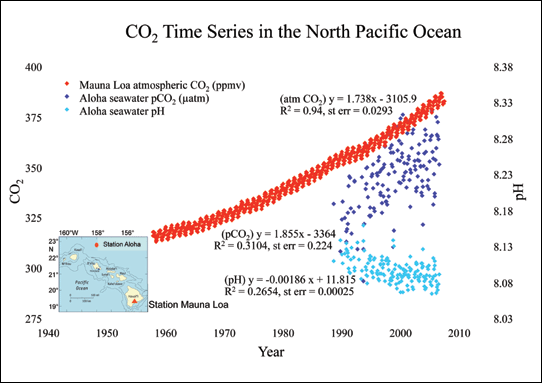
Projections based on these numbers show that by the end of this century, continued emissions could reduce ocean pH by another 0.5 units. Shell-forming animals including corals, oysters, shrimp, lobster, many planktonic organisms, and even some fish species could be gravely affected. Also, is the fact that as the oceans continue to absorb more CO2, their capacity as a carbon storehouse could diminish. That means more of the carbon dioxide we emit will remain in the atmosphere, further aggravating global climate change.
Scientific awareness of ocean acidification is relatively recent, and researchers are just beginning to study its effects on marine ecosystems. But all signs indicate that unless humans are able to control and eventually eliminate our fossil fuel emissions, ocean organisms will find themselves under increasing pressure to adapt to their habitat’s changing chemistry or perish. When atmospheric carbon dioxide dissolves in the oceans it forms carbonic acid, which in turn has a negative impact on marine life and Algae.
Laboratory studies show that as seawater acidity increases, the calcium carbonate shells and skeletons of many marine species, such as hard corals, sea urchins, and stony seaweeds, begin to corrode.
Four leading marine experts delivered this grim prognosis recently at the annual meeting of the American Association for the Advancement of Science in San Francisco, California.
The scientists stressed that increased ocean acidity is one of the gravest dangers posed by the buildup of atmospheric CO2.
“Ocean chemistry is changing to a state that has not occurred for hundreds of thousands of years,” said Richard Feely of Seattle’s Pacific Marine Environmental Laboratory.
Already, Feely said, ocean acidity has increased about 30 percent since industrialization began spurring harmful carbon emissions centuries ago. Unless emissions are reduced from current levels, an increase of 150 percent is predicted by 2100.
So what does this mean for me you might ask? It means that we are inconsiderate as tenants of this planet, which is our “Landlord”, and we are trashing it…
We act like gangs we complain about and who destroy everything in their path. Is it too late? No! But it surely is time to wake up and not be so nonchalant about all the environmental issues today. Our life and the future of humanity depend on it. It is that serious!
This means that all of life in the Ocean and on Earth will be affected. How? Here it is:
If you are a science fiction fan, you will have heard humans referred to as carbon based life forms. In fact, all living creatures on Earth are based on carbon. Carbon is the basic element in our web of life.
So where is the problem? Every time we burn fossil fuels such as gas, coal or oil, carbon dioxide is released into the atmosphere. In a natural carbon cycle, carbon dioxide is re-absorbed by plants and trees. However, we are burning fuels where the carbon dioxide has been trapped under the earth’s surface for millions of years, and we’re doing it so quickly that plants and trees that are alive now have no chance of soaking it up (and it doesn’t help that we’re cutting down rainforests as well).
The effect of all this extra carbon dioxide in the atmosphere is that the overall temperature of the planet is increasing (global warming). The climate is changing in unpredictable ways (from floods and hurricanes to heat waves and droughts). To try and reduce the risk of ever more extreme weather, we need to reduce how much fossil fuel we are burning. This isn’t easy.
 Carbon Dioxide harms ocean life. The ocean has absorbed almost half of the carbon dioxide that humans have made in the last 200 years, which is about 118 billion metric tons. Carbon dioxide harms coral and free-swimming algae. The carbon dioxide makes it more difficult for coral to form their outer shells. How does the ocean gather carbon dioxide? When the currents stir the ocean by pulling deep water from the surface, where the carbon dioxide gets trapped. Carbon dioxide causes the greatest threat to the species that live on the top of the ocean water. That’s because that is where the currents gather the carbon dioxide. Cars can also cause pollution to the ocean, not directly, but indirectly. The car’s exhaust (which is carbon dioxide) is evaporated, which can cause acid rain and would eventually make its way into the ocean. It’s the same thing with boat gasses and agricultural pollution (except with pesticides).
Carbon Dioxide harms ocean life. The ocean has absorbed almost half of the carbon dioxide that humans have made in the last 200 years, which is about 118 billion metric tons. Carbon dioxide harms coral and free-swimming algae. The carbon dioxide makes it more difficult for coral to form their outer shells. How does the ocean gather carbon dioxide? When the currents stir the ocean by pulling deep water from the surface, where the carbon dioxide gets trapped. Carbon dioxide causes the greatest threat to the species that live on the top of the ocean water. That’s because that is where the currents gather the carbon dioxide. Cars can also cause pollution to the ocean, not directly, but indirectly. The car’s exhaust (which is carbon dioxide) is evaporated, which can cause acid rain and would eventually make its way into the ocean. It’s the same thing with boat gasses and agricultural pollution (except with pesticides).
As human activities (like driving) have pumped carbon dioxide into the air, the oceans have absorbed a large portion of this gas. Richard Feely of the National Oceanic and Atmospheric Administration and his colleagues wanted to know what the effects might be on certain ocean animals that are sensitive to the chemistry of the water they live in. Many mollusks, corals, and single-celled creatures called foraminifera and coccolithophorids use ingredients in seawater to build their shells and other hard parts. Specifically, they pull “carbonate” ions out of the water and make a hard material called “calcium carbonate.”
As you might guess from the name, carbon plays an important role in the chemical reactions that allow these animals to make their homes. But, if the amount of carbon dioxide in the seawater increases above a certain level, these conditions lead to a different set of reactions that don’t produce carbonate ions.
In parts of the ocean that don’t have enough carbonate ions, calcium carbonate shells start to dissolve.
Feely’s research team used new ocean chemistry measurements to estimate how fast calcium carbonate is dissolving in the world’s oceans. In the 16 July 2004 issue of the journal Science, the researchers predict that if carbon dioxide emissions continue to increase, ocean areas where these animals have trouble building their homes could expand.
This trend would probably begin with colder surface waters at higher latitudes, which might mean that the mollusks and other shell-forming animals living there would have to move to lower latitudes. If the trend continued, the areas where calcium carbonate dissolves could keep moving closer to the equator, the researchers say.
These predictions don’t necessarily have to come true. If humans can change our activities so that we don’t put so much carbon dioxide in the atmosphere, then we might be able to modify our impact on ocean chemistry.
Ocean Dead Zones Likely To Expand: Increasing Carbon Dioxide And Decreasing Oxygen Make It Harder For Deep-Sea Animals To Breathe.
Climate Change Could turn part of the Oceans “Oxygen-Free” called “dead zone”.
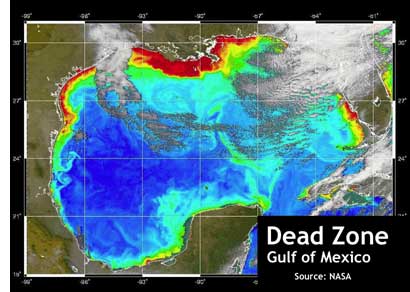
Dead zones are hypoxic (low-oxygen) areas in the world’s oceans, the observed incidences of which have been increasing since oceanographers began noting them in the 1970s.
Aquatic and marine dead zones can be caused by the process of eutrophication, triggered by an excess of plant nutrients (nitrogen and phosphorus) from fertilizers, sewage, combustion emissions from vehicles, power generators, and factories.
The finding, by Rudolf Wu, Ph.D., and colleagues at the City University of Hong Kong, raises new concerns about vast areas of the world’s oceans, known as “dead zones,” that lack sufficient oxygen to sustain most sea life. Fish and other creatures trapped in these zones often die. Those that escape may be more vulnerable to predators and other stresses. This new study, Wu says, suggests these zones potentially pose a third threat to these species — an inability of their offspring to find mates and reproduce.
Whereas some coastal dead zones could be recovered by control of fertilizer usage, expanded low-oxygen areas caused by global warming will remain for thousands of years to come, adversely affecting fisheries and ocean ecosystems far into the future.
Professor Gary Shaffer of the Niels Bohr Institute, University of Copenhagen, who is the leader of the research team at the Danish
Center for Earth System Science (DCESS), explains that “such expansion would lead to increased frequency and severity of fish and shellfish mortality events, for example off the west coasts of the continents like off Oregon and Chile”.
He adds that “if, as in many climate model simulations, the overturning circulation of the ocean would greatly weaken in response to global warming, these oxygen minimum zones would expand much more still and invade the deep ocean.” Extreme events of ocean oxygen depletion leading to anoxia are thought to be prime candidates for explaining some of the large extinction events in Earth history including the largest such event at the end of the Permian 250 million years ago.

We at GreenDustries are conscious of the effect of our industry on our planet. We are the “champions of the 3R’s, Recycle, Reduce and Reuse”
GreenDustries stands for Green Industries, we care…..
Visitors: 90954



No comments yet.